I find them stuffed into the toes of a new pair of sneakers. I find them wedged into a sheaf of seaweed snacks. I find them in the over-inflated bag that contains my new inhaler, and in the vacuum-sealed one puckered around my kids’ 3D printing filament. “DO NOT EAT,” they all admonish me, and I find myself slipping them under the top layer of the garbage already in the trash can, as if my kids wouldn’t be able to control their urge to taste whatever is inside these tiny white pouches. Silica gel packets are everywhere, their presence seemingly the only thing keeping our packaged food crispy and our belongings free of mildew. How on earth did they all get here? Is silica gel taking over the world?

Tear its little Tyvek wrapping, and spill a packet of glassy silica gel beads into the palm of your hand; they won’t hurt you. They are made of the same stuff as sand: “Silica” means “silicon dioxide,” which is the primary component of most drinkware, windshields, and the screen of whatever electronic device you’re reading this on. But glass has a density of around 2500 kilograms per cubic meter, and crystalline silicon dioxide (quartz) is around 2650. Silica gel, on the other hand, is more like 700 kilograms per cubic meter. It may look fully dense, but in fact it’s shot through with countless tiny pores. If your windowpane is like a thin sheet of solid ice, then a silica gel bead is like a tiny snowball.
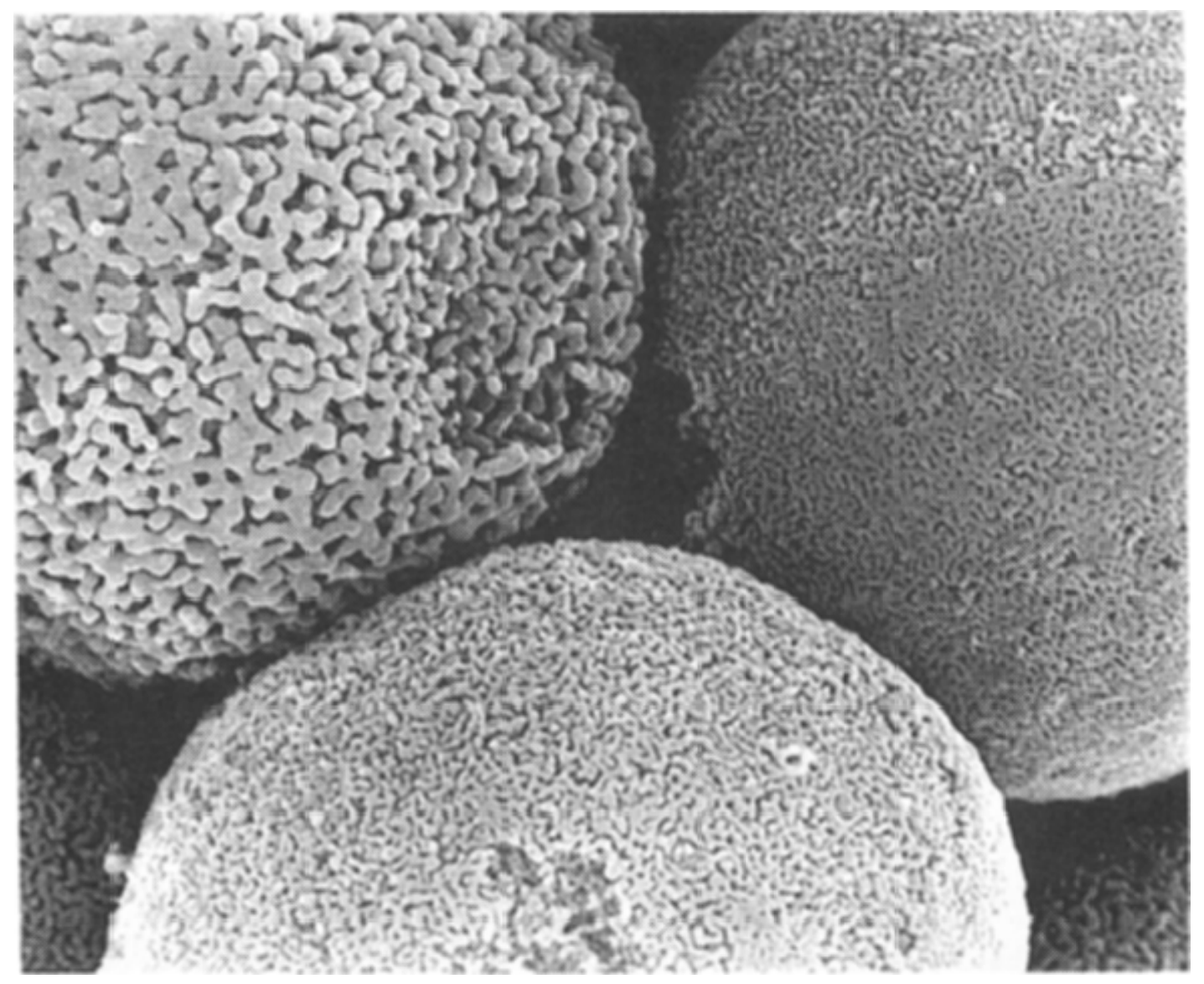
Zoom in on a silica gel bead with a scanning electron microscope, and its smooth surface turns discontinuous, riddled with voids about 2.5 nanometers across (roughly the diameter of a strand of DNA). This microstructure gives silica gel radical properties. The silica gel packets in my kids’ seaweed snacks are just a little bit bigger than postage stamps, and have a total mass of about a gram. That single gram of silica gel could have an internal surface area of eight hundred square meters—the size of almost two basketball courts.
These factors allow silica gel to adsorb up to 40% of its own weight in water vapor, through a process called capillary condensation. When humid air migrates into the pores of a silica gel bead, its vapor pressure increases, causing water to condense onto the silica gel’s internal surfaces. At the risk of anthropomorphizing water, it’s as if it prefers to be a liquid, stuck within the silica gel’s tiny capillaries, than a vapor, carried along with whatever else is in the air.
If you find a packet of silica gel in an imported snack or the pocket of a new jacket, it’s probably there to filter water vapor out of air. Like most filters, there is a limit to how much water a piece of silica gel can hold; its internal surfaces are finite in size, and as a result there is a finite amount of humidity that a given packet of silica gel can adsorb. Luckily, silica gel vendors offer nifty calculators so that their customers can size their silica gel packets to the volumes of air they wish to dry out—and how dry they want the air to be. If you wanted to desiccate the air inside of a child’s balloon, you’d calculate its volume (let’s call it 14 liters), then make some assumptions about its temperature and humidity (around 35°C, 75% RH), then decide what you want its final relative humidity to be (let’s bring it down to 20% RH). Run these numbers and you’d find you needed to pull about 0.3 grams of water out of the air in your balloon. This can be done, pretty reliably, with a one- or two-gram packet of silica gel, slipped into the balloon just before you blow it up.
Of course, different applications will entail different goals. The bag that holds your potato chips is not completely impermeable to moisture, and if you’re going to ship it across an ocean and then let it sit on a shelf for a few months, you should expect some additional water vapor to find its way in. There are also applications in which silica gel is used not so much to dry something out but to maintain a particular equilibrium humidity. In the art world, silica gel packets are slipped into exhibit cases and used for their “buffering capacity,” helping maintain a stable (and relatively high) humidity inside the case while conditions in the rest of the gallery might vary.
While glass and glassy substances have been used by humans for many thousands of years, it wasn’t until the early twentieth century that Walter Patrick, a researcher at Johns Hopkins, developed and patented an efficient way to create silica gel. He did this by mixing a substance called “water glass” with an acid. Water glass—a fascinating material in itself, as it is essentially water-soluble glass—is an alkaline compound containing sodium oxide and silicon dioxide. When mixed with an acid its silicon dioxide precipitates, linking into the matrix now known as silica gel. The silica gel—glassy and hard—is then washed and dried to remove excess acid, salt, and water.
Silica gel became commercially available within five years of Patrick’s invention, through a deal with the Davison Chemical company. But according to this academic paper, which analyzes how discoveries at Johns Hopkins impacted the local economy, “the transition from the laboratory to the commercial world was a long and arduous one... Much development work had been needed to develop the original academic breakthrough, and new applications had to be found for the product before it became a commercial success.”
But a commercial success it eventually became. In 1927, Davison built a silica gel factory in Baltimore, and by 1930 they claimed to have applied for or received over three hundred patents on the stuff. The company was acquired by W.R. Grace in 1954, and Grace continues to manufacture specialty silica gel products at that same location.
To learn about the silica gel industry today, I spoke with Demetrius Michos, a PhD chemist who has worked at Grace for over thirty years. He assured me that I “could not go about my day without touching Grace’s products,” and went on to repeatedly surprise me with obscure and curious applications for silica gel. But they've mostly moved up-market from the little envelopes in our consumer packaged goods. Grace is big in the silica gel business, and they sell a lot of other silica products, but they don’t seem very interested in silica gel desiccant packets specifically.

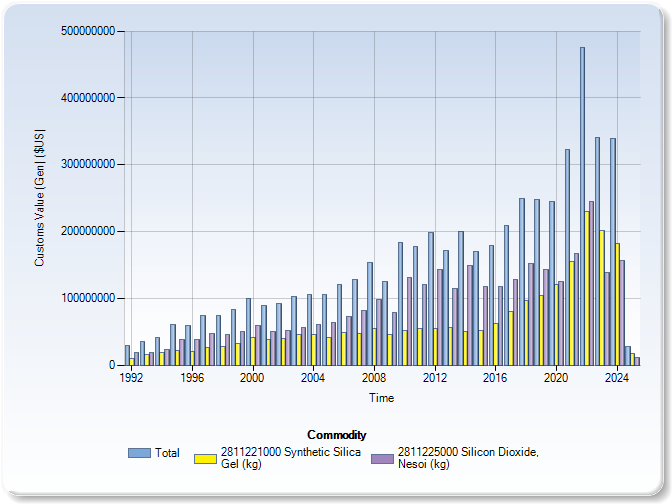
So I went looking for imports. The Census Bureau’s import data goes back to 1992, and shows rising—and accelerating—imports of both silicon dioxide and silica gel. Total imports peak in 2022, and are currently about ten times their 1992 levels. This is interesting, but it does not answer my question about desiccant packets. Sure, we may be importing more silica gel today than we did in the early nineties. But we’re also importing a lot more shoes than we did in the nineties, and it's not like I find pairs of shoes slipped into every third thing I buy.
After a round of mostly fruitless phone calls, I finally spoke to John Perona, a sales rep at a company that sells, among other things, silica gel desiccant packets. He started by pointedly questioning my intention of writing anything about silica gel at all, then told me that I was “getting into the weeds. Just look at how things ship around the world, and you can understand the problems that silica gel packets are trying to address.”
The reasons behind the increase in silica gel imports, he went on somewhat reluctantly, were simple: “No one wants a silica gel factory in their backyard.” Only specialty silica gel products are manufactured in the US today; the packets that I find in my snacks and pharmaceuticals are either made overseas or, in some cases, assembled in the US from imported silica gel beads.
Then John reminded me that a couple decades ago there were hundreds of thousands of manufacturing jobs on a single mile of road, a half-hour from my house. If I lived in Brooklyn then, I could have purchased goods from those factories. They would experience few, if any, swings in temperature and pressure on their short journey from factory to home, and even if they did, their packaging probably would have let excess humidity ventilate off.
The farther you ship a product—the longer it takes to go from the factory to the customer’s hands, and the more temperature and pressure cycles it experiences during that time—the more you need to control humidity inside of its airtight packaging. Silica gel is a cheap, easy, and reliable way to do so. In this sense silica gel sits alongside containerized shipping, and stretch wrap, and bills of lading: It is a technology without which we’d have a much harder time maintaining global supply chains.
Desiccant packets haven’t actually taken over the world—globalization has.
So we seal our seaweed snacks, and our inhalers, and our 3D printer filament inside airtight plastic bags—then ship them across the world. We could, as one engineer I spoke to suggested, fill all these packages with dry, inert gas first. But little Tyvek bags, slipped inside right before they’re sealed up, are quite a bit easier.
Update: We published a follow-up to this piece here; it contains a bunch of fascinating miscellany about silica gel.
Thanks! To Nick Fountain for suggesting the topic, and to Demetrius Michos, Sharyn Nerenberg, John Perona for educating me on it. Thanks to Brad Avenson for suggesting (facetiously) that we fill our products & packaging with “dry, inert gas,” and a big thanks to the Supporters and Members of Scope of Work, who make it possible for me to spend a week immersing myself in a little corner of the infrastructural world.




